Any CASA has to demonstrate a high degree of ‘parity’ with measurements made by the haemocytometer as ‘gold standard’. This is not the same as correlation and any manufacturer of a sperm counting device which only shows correlation with the gold standard should be disregarded and must demonstrate sample by sample comparison. Fundamentally if CASA cannot count sperm properly there are implications for the evaluation of other parameters as it reflects the inability to accurately identify objects under the microscope. Of course, during validation not every measurement will turn out the same for both methods just like they do not when you provide repeated haemocytometer readings as there are inherent errors associated with most methods which cannot be avoided. This is why in general we suggest that any unit trying to validate its CASA against the haemocytometer follows strict guidelines for reducing error and make use of the protocols listed in the WHO manual. Any taking of shortcuts or use of an alternative chamber to the haemocytomter will not be appropriate for validation purposes. Done carefully results similar to those shown in the figure below.
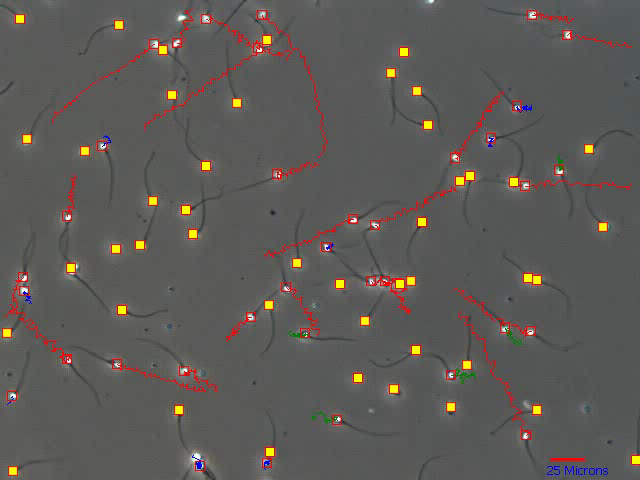 How can we validate motility? We are under no illusions that this is difficult since there is no traceable standard which we may use as a basis for calibration. If as we are to believe from the literature, motility grades should be based on swimming speeds then at least using the videoeach sperm. Essentially the still image from the below which represents a 1 second video loop can be played and the track length verified against the 25 micron scale (bottom right).
How can we validate motility? We are under no illusions that this is difficult since there is no traceable standard which we may use as a basis for calibration. If as we are to believe from the literature, motility grades should be based on swimming speeds then at least using the videoeach sperm. Essentially the still image from the below which represents a 1 second video loop can be played and the track length verified against the 25 micron scale (bottom right).
Download Validation Protocol PDF
Highly repeatable sperm concentration data over 8 year period. Figure 1 . shows data from 2 centres using first prototype of Sperminator the forerunner of SAMi. Figure 2. Shows current data for SAMi with an overall 1% difference between methods.
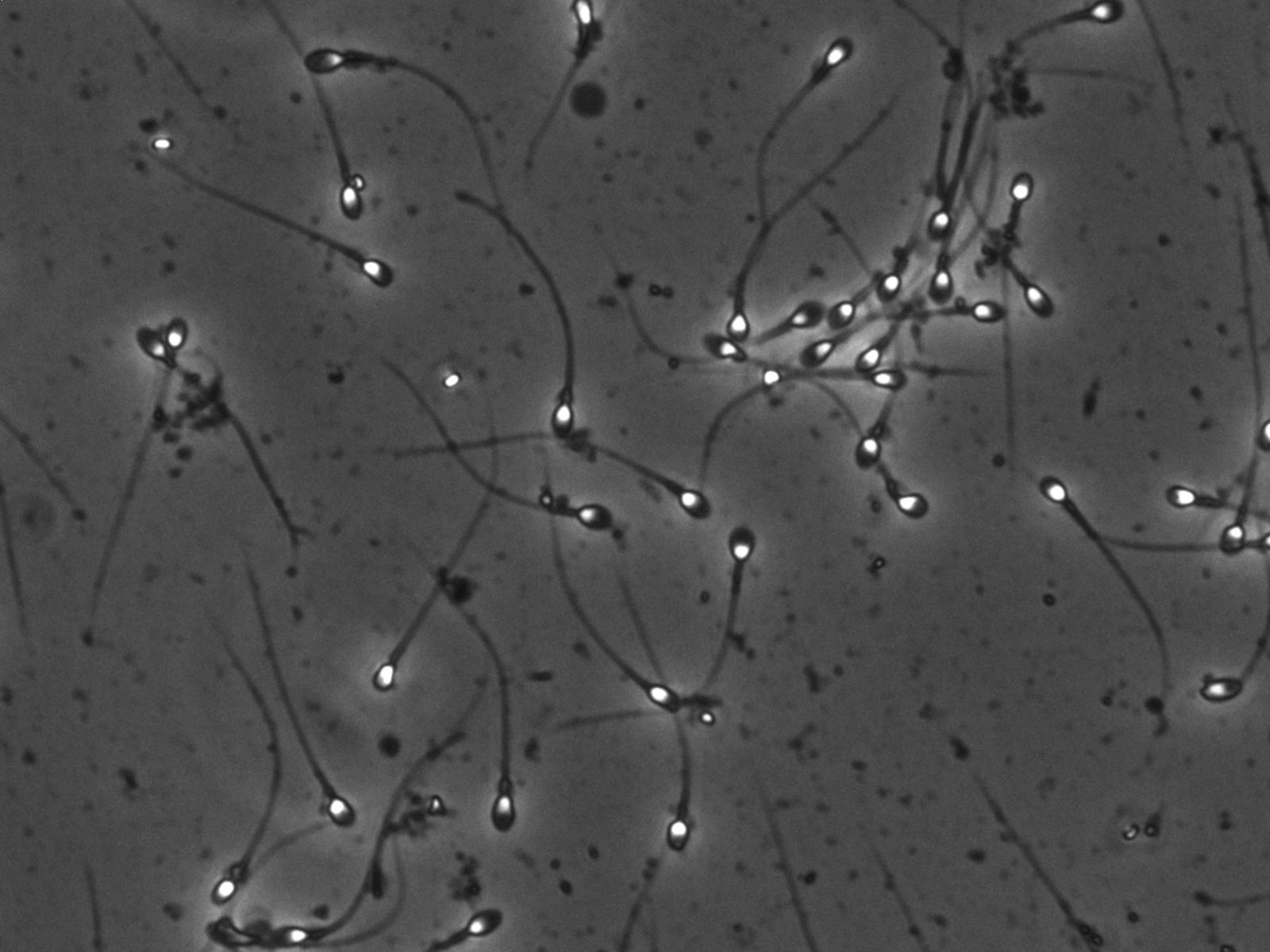 As with any methods of laboratory analysis there are always limitations of a CASA and understanding these will help users get the best out of the system. First and foremost, problems with image quality and especially microscopy will affect object recognition and therefore evaluation of every parameter. It is surprising just how many centers do not offer basic microscopy training and complain of poor image quality on their CASA but simply have poorly adjust phase contrast. Images such as the one shown below showing good contrast between background and white sperm heads are ideal.
As with any methods of laboratory analysis there are always limitations of a CASA and understanding these will help users get the best out of the system. First and foremost, problems with image quality and especially microscopy will affect object recognition and therefore evaluation of every parameter. It is surprising just how many centers do not offer basic microscopy training and complain of poor image quality on their CASA but simply have poorly adjust phase contrast. Images such as the one shown below showing good contrast between background and white sperm heads are ideal.
or this reason the following link provides information on basic adjustment of the phase optics of one of the microscopes we commonly recommend: http://meijitechno.com/how-to-center-the-phase-contrast-and-dispersion-staining-condenser-for-the-mt6830
A clear limitation to its use in routine practice is related to the inherent biological variation in semen composition and the effect that this has on the image recorded by the video system. Indeed, the image obtained by the microscope and camera is key to the satisfactory detection of sperm, even to the level of how grey is the background field. Therefore analysis of, for example washed sperm suspensions will often be very clear and consistent with excellent sperm detection but, adjustments must be made for any system to account for the effect that seminal plasma and other contaminants may have on image quality.
The operator must have some idea of how all of these factors may affect the eventual outcome. Sperm must be allowed to settle adequately before evaluation takes place in order that immotile sperm are not inappropriately classed as motile. The presence of non-sperm cells or debris will in some cases be erroneously identified as sperm and must have trackers removed within review screen, if they are not excluded adequately by ‘gaiting’. Agglutination and aggregation will affect the degree of homogenisation and therefore accuracy of any analysis, be it manual or automated and in the worst-case scenario no reliable analysis can be made no matter what method is used.
Accuracy will also be affected by having too few sperm evaluated, too many to evaluate in a single field or indeed the motility is so vigorous as to cause excessive collisions either sperm with other sperm or sperm to non sperm cells.
All testing has been completed using a sample minimum of 200 sperm in line with WHO recommendations and have clearly provided adequate results. However it is also patently clear that increasing the cell number per analysis is permitted with automation and the following statements should be made clear to the user.